Mitochondria are often linked to Parkinson’s disease. Damage to these tiny components of the cell is considered a key in the development of Parkinson’s-like changes in the brain.
So, what are mitochondria and how do they contribute to Parkinson’s disease?
Keep reading this article to find the answer!
Parkinson’s is a neurological condition that develops when there is a massive death of a certain group of cells that produce dopamine, a chemical in the brain that is responsible for controlled body movement.
The typical symptoms of the disease include slowness of movement, tremor of hands and other parts of body, rigidity, and balance issues.
Although the exact cause of Parkinson’s disease is still unknown, researchers have identified several abnormal features in the brain cells that contribute to the disease changes. One such feature is the abnormal mitochondria.
What are Mitochondria?
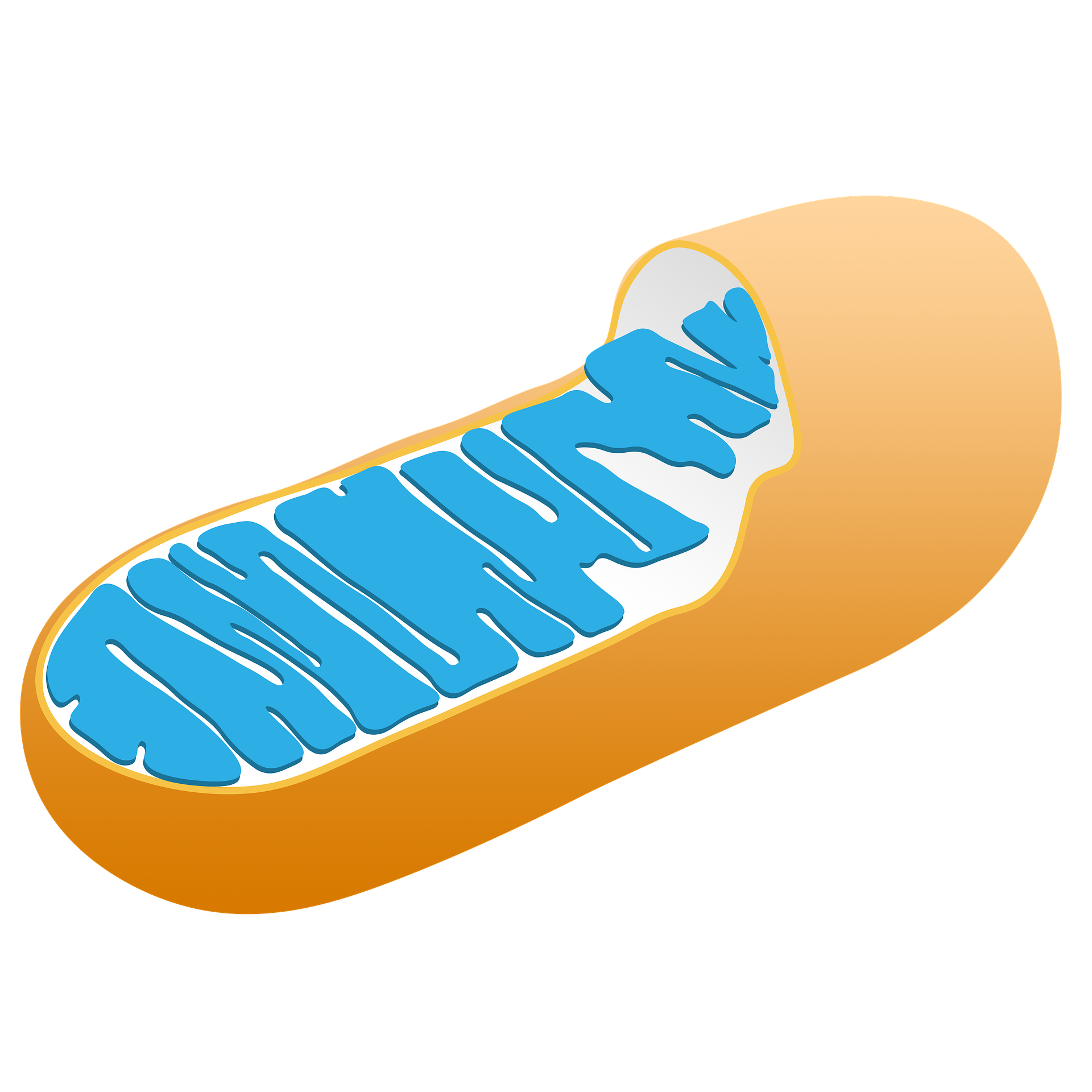
Mitochondria are tiny structures inside the cell that look like rods. They produce most of the cell energy; this is why they are often known as the powerhouses of the cell. Brain cells have a high amount of mitochondria as they need more energy.
An individual mitochondrion is made up of 3 main parts:
- Outer-membrane
- Inner-membrane
- Matrix
The outer membrane forms the outer layer and consists of small pores. These pores allow the passage of small molecules and nutrients. The inner-membrane is more complex than the outer-membrane. It is the site for a large number of proteins required for energy production. Matrix is the compartment enclosed by the inner-membrane. This compartment contains mitochondrial DNA, enzymes, and other small molecules that facilitate the process of energy production.
The energy production by mitochondria is not simple. It’s a stepwise process that mainly requires the participation of five major protein complexes that reside within the inner-membrane. These are designated as Complex I to Complex V ( shorty C-I to C-V). During this process, the electrons generated from the oxidation of fats and carbohydrates are passed through C-I and reach C-IV to form water.
The transport of electrons is coupled by a proton pump that generates electrochemical gradient across the membrane. Using this gradient, the C-V generates the energy which is stored in a molecule called ATP (adenosine triphosphate).
How does Mitochondria Contribute to Parkinson’s?
Research in the last few decades has confirmed that there is a direct link between mitochondria and Parkinson’s disease. And it’s now generally accepted that mitochondria play a big role in the development of Parkinson’s disease.
There are two main ways through which mitochondria contribute to Parkinson’s disease. One is the mutations in genes linked to mitochondria. The other is through the generation of reactive oxygen species that cause damage to vital components (like DNA, protein, and lipids) of brain cells, especially those involved in dopamine production.
1. Mitochondria-related Gene Mutations
Genetics account for 10% of Parkinson’s cases. Although the rest 90% cases have no clear-cut genetic origin, researchers think that they may also involve some genetic components.
Researchers have identified several Parkinson’s-related genes that are directly linked to mitochondria. These include PINK1, PARKIN, DJ-1, SNCA, LRRK2, ATP13A2, and VPS35. Among these, the PINK1 and DJ-1 are mitochondrial specific genes. While the remaining genes are predominantly located outside the mitochondria, they still directly influence mitochondrial integrity.
PINK1 (Phosphatase and Tensin Homolog (PTEN)‐induced Kinase 1)
The protein product of this gene is located within the inner-membrane of mitochondria. It changes its location and transfers to the outer-membrane when mitochondria have been damaged.
People carrying a mutation in this gene can develop Parkinson’s usually before the age of 40s. Some can develop it after that. The progression of the disease is slow and the patient shows a good response to medication.
PARKIN
This was the second gene that was identified to be linked with Parkinson’s disease. And probably the most widely studied gene in Parkinson’s research. The protein product of this gene is located outside the mitochondria. But it translocates to the outer-membrane of mitochondria when there is an abnormal condition. It works together with PINK1 and collectively forms a pathway to remove mitochondria that are no longer needed.
Mutations in this gene account for most of the genetic causes of Parkinson’s disease. People with Parkinson’s caused by mutation in this gene are young. Some can develop the disease at old age.
DJ-1
There was a controversy about the location of the protein product of this gene. But now most researchers agreed that it’s of mitochondrial origin. Its main function is to protect the cell from the oxidative stress insult, which is mostly caused by damaged mitochondria.
Mutations in DJ-1 have been found to cause early-onset Parkinson’s. In most cases, the disease symptoms may appear before age 45.
SNCA (Synuclein Alpha)
This was the first gene that was reported to cause Parkinson’s disease when mutated. Its exact function in the cell is still unclear. Some researchers suggest that it may have a role in dopamine metabolism.
Mutation in the SNCA gene is known to form abnormal protein structures that are toxic to dopamine cells. These abnormal structures are called Lewy body, which is the pathological hallmark of Parkinson’s disease.
People having Parkinson’s disease as a result of a mutation in this gene are rare and usually are under the age of 50s. These patients also show signs of dementia and learning problems.
LRRK2 (Leucine-rich Repeat Kinase 2)
Mutations in LRRK2 directly compromises the function of mitochondria and ultimately contributes to the development of Parkinson’s disease. In fact, mutations in this gene are the most common genetic cause of Parkinson’s disease.
The product of this gene has multiple functions in the cells. In regard to mitochondria, it maintains the dynamics and quality control mechanism.
The effects of mutation usually appear after the age of 50s. These effects mostly appear in the form of postural instability and gait difficulty.
ATP13A2 (Probable Cation-transporting ATPase 13A2)
This is a newly identified Parkinson’s-related gene that produces a protein important for the clearance of damaged mitochondria.
Mutation in ATP13A2 gene is linked to a rare form of Parkinson’s known as Kufor-Rakeb syndrome. People carrying a mutation in this gene develop the disease signs at a young age. The youngest patient reported to carry a mutation in ATP13A2 was a 12 years old Lithuanian boy.
In addition to other typical symptoms, a patient may also show dementia.
VPS35 (vacuolar protein sorting 35 ortholog)
Mutations in VPS35 cause severe abnormalities in the structure of mitochondria that in turn lead to neuronal cell death and develop Parkinson’s disease. This is also a newly identified Parkinson’s-linked gene and researchers are trying to understand how mutations in this gene contribute to the death of dopamine producing cells.
However, it’s now known that its mutations cause a late-onset form of Parkinson’s disease.
2. Source of ROS Production
Mitochondria are the main source of reactive oxygen species (ROS), which are highly reactive chemical species derived from molecular oxygen. They are generated as a result of the abnormal processing within the inner membrane of mitochondria. Specifically, it happens when electrons skip a normal sequence of reaction steps (as discussed above) and react with free oxygen. Impaired mitochondrial functions further increase the levels of ROS.
Low levels of ROS are not of concern. Infact, they participate in various physiological processes in the cell. However, when generated at abnormally higher levels, they become a threat to the cell.
Cells have evolved numerous antioxidant defense systems to counteract the negative effects of ROS. But sometimes, these defense mechanisms are insufficient to keep ROS below the toxic levels. This results in a condition called oxidative stress.
Oxidative stress is strongly linked to Parkinson’s disease. It destroys the dopamine producing cells and ultimately leads to Parkinson’s like changes in the brain. This is supported by numerous postmortem studies where brains from patients were found to have increased levels of oxidative stress.
